A Guide to IEC 60601-1-8 and Medical Alarm Systems
The design of medical electronic equipment seems like a straightforward task, until you get into the details. Remember, as you know, this type of equipment is used in situations that range from home health care to surgical suites. Products used in the medical arena must perform, must be reliable, and must be effective, since they are frequently part of a life-saving process.
Many types of medical electronic equipment incorporate some type of alarm to indicate patient problems, equipment failure, or power interruption. In most cases these alarms are either audible or visual, but research has shown that audible signals can provide a strong sensory cue to establish the awareness of a situation. Simply stated, we can shut our eyes to block out the visual, but turning off our ears is a bit more difficult.
The number of alarms used on the equipment in a medical setting, as well as the amount of new medical electronic equipment being developed is also growing rapidly worldwide. The problem is, ongoing surveys of healthcare personnel who operate or rely on medical electronic equipment continue to indicate displeasure with the alarm signals on the devices, including concern with their loudness, distraction due to multiple signals, and recognition of the critical ones. These challenges, coupled with the demands of patient safety, mean additional regulation. It should come as no surprise then, that the alarms used in medical electronic equipment are also subject to relatively strict technical standards and guidelines in the form of the IEC Standard IEC 60601-1-8. In this blog, we will review the general outline of IEC 60601-1-8 and the key requirements given for audible alarms in medical equipment as well as providing example medical buzzer tones.
What is IEC 60601-1-8?
The International Electrotechnical Commission (IEC), a European-based standards organization, published the IEC 60601 international consensus technical standard covering all medical equipment requirements some time ago. A subset of the standard is IEC 60601-1-8, which deals with medical alarm systems. This portion of the standard is officially titled: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems.
IEC 60601-1-8 Requirements for Audible Alarms in Medical Equipment
If you are a designer of electronic medical devices or systems, the alarm components you need to specify for patient and device monitoring are indeed critical, and the process may take a bit more planning than you thought. Fortunately, however, there is guidance in place to help you.
The IEC 60601-1-8 standard was developed and continues to be updated to help regulate alarm design and prevent confusion in medical settings where several alarms and ongoing signals may be sounding at the same time. It is a relatively lengthy technical document that sets a framework for the alarm sounds that medical electronic equipment should make in different healthcare situations. The standard addresses the design of the alarms that can be used in medical equipment by doing the following:
- Defines the medical conditions that should trigger an alarm.
- Classifies alarms as either low, medium, or high priority.
- Defines the alarm pulse frequency range, pulse shape, rise/fall time, and signal burst pattern.
- Prescribes alarm sound levels in decibels as they relate to priority.
- Sets a maximum amplitude difference between the alarm pulses.
- Separates physiological alarms, or those related to patient condition, from technical alarms, or those related to equipment status.
- Discusses the temporary silencing (muting) of alarms by healthcare personnel.
- Allows for specific melody alarm tones for certain applications, including general, cardiac, artificial perfusion, ventilation, oxygenation, temperature/energy delivery, fluid/drug delivery, and equipment or supply failure.
From a technical standpoint, and to give you an idea of how technically focused the document is, here are some of the specific metrics that the standard assigns to medical alarms:
- The alarm frequency must be between 150 Hz to 1,000 Hz and must be one of four harmonics with the greatest sound level.
- There must be a minimum of four frequency peaks between 150 Hz and 4,000 Hz.
- The sound level of the greatest four frequency peaks between 150 Hz and 4,000 Hz must be within 15 dB of each other.
While specific in noting that all medical equipment must include at least one set of audible warning sounds that meet the requirements of the standard, it also allows for additional sound sets to be built into the equipment, including music, voice, or, outside of audio, visual alarms. The intent is that these alternate alarms may be easier to learn and identify by healthcare personnel. Voice alarms are frequently used in aircraft cockpit pilot warning systems.
Compliance with the IEC 60601-1-8 standard for medical equipment alarms is mandatory in the U.S., Canada, and the EU to promote evidence of device safety and performance.
A Word About Components Used in Medical Alarm Systems
Alarm system components for medical electronic equipment can range from buzzers, bells, or sirens for relatively uncomplicated situations, to audio speakers or transducers for voice or music tones, and to visual indicators to complement audio tones. Deciding what makes sense for your design will obviously hinge on the system you are designing for, the setting in which it will be used, the skill or learning ability of the potential operator personnel, and your budget.
 Example of CUI Devices’ medical buzzer
Example of CUI Devices’ medical buzzerThere may also be a discussion among your design team about the complexity of the alarm system required by your device or system in order to get the desired response needed for a particular healthcare setting. For example, will a buzzer suffice in an operating room setting, or does it need to be coupled with a visual indicator?
The reality of designing alarms for medical applications is that you are not just doing device or systems engineering. You are working with information design and communication, along with accommodating the complex human factors that exist in the healthcare environment. Alarms not only have to be noticed, they also must be learned and recognized by the healthcare staff.
Using Electrical Buzzers as Medical Equipment Alarms
Electrical buzzers are straightforward audio signaling devices that are available in many configurations, footprints, frequency ranges, voltages, drive currents, sound pressure levels, and price points. They convert electrical signals into sound that, depending on the device, can vary by volume, tone, frequency, and pulse. Buzzers may also be called audio alarms, audio indicators, or sounders.
Generally powered by dc voltage, buzzers come in two types:
- Electromechanical (or magnetic) – uses a magnet, oscillator, and vibrating diaphragm to generate sound.
- Piezoelectric – uses ceramic piezo materials that deform when a current is applied, resulting in sound generation.
Electromechanical buzzers are traditional components in that they use a magnetic field produced by an electric current. They typically operate at lower voltages but higher drive currents than piezoelectric buzzers. Piezoelectric buzzers operate at larger frequency ranges than electromechanical types due to their more linear relationship between input frequency and output power level. Piezo buzzers also have larger sound pressure levels (SPL) and higher resonant frequencies than electromechanical buzzers.
Using a buzzer as an audible medical alarm can offer ease and elegance of design-in, high performance, low cost, and high reliability – as long as you comply with the guidelines of the IEC standard discussed. To make this aspect of medical alarm design simpler, manufacturers, such as CUI Devices, have created specific medical buzzers that meet those IEC 60601-1-8 guidelines.
These specialized buzzers create tones that correspond exactly to those requirements set out by the IEC standards for different applications, reducing the steps required by you as the designer in creating a system. As examples, these include the following tones as indicated by Table G.4 within the guidelines (click the links below to listen to the specific tones):
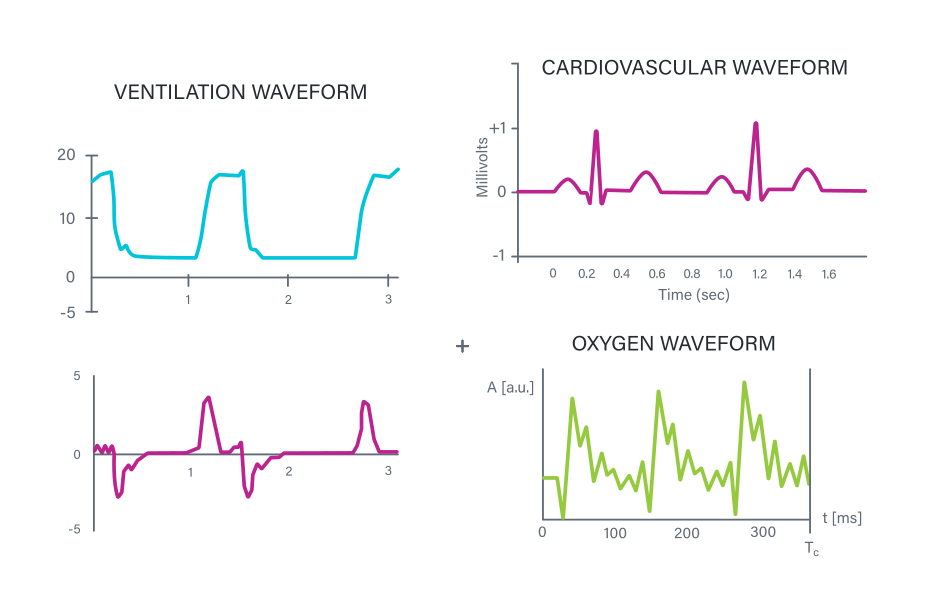
- General tone: A more generic tone for non-specific applications.
- Cardiovascular tone: A heartbeat sound with specific “lup-dup” sequences.
- Ventilation tone: An inhalation sound with a pause followed by an exhale.
- Oxygen tone: An irregular sound with stylized dripping or saturation sounds.
Using Speakers in Medical Equipment
IEC 60601-1-8 focuses a considerable amount of attention on the communication challenges, the required waveforms, and the environments in which medical equipment is found. However, it does not provide a significant amount of information on the actual physical equipment to be used in medical equipment applications. As such, while buzzers are an extremely popular option due to their low power requirements, high power to sound-level ratio, and general robustness, speakers can also be used in medical equipment.
Speakers may not be as power frugal as buzzers, but they make up for it with increased flexibility in what sounds they can reproduce and, in some cases, improved sound quality. For example, if the medical equipment should also provide some sort of spoken audio alert in addition to the alarm, a buzzer would be unable to recreate a human voice, thus making a speaker the appropriate choice.
You should be aware that speakers are more prone to “popping” noises when exposed to abrupt voltage changes and the IEC standards warn against these pops. This can be avoided with appropriate waveform shaping, careful software development, and hardware precautions against transients. As long as you follow these steps and select a speaker that can withstand anticipated environmental conditions, speakers are an excellent option for medical alarms. CUI Devices has created specific medical speakers designed to meet the IEC 60601-1-8 guidelines.
Conclusion
The use of audible alarms in medical equipment is expanding as both the number of alarms per device increases and the quantity of devices being used in healthcare settings expands. Audible medical alarms should help healthcare personnel easily identify the onset of an alarm condition, differentiate between a patient problem and an equipment problem, communicate the urgency of the response that may be needed, and easily indicate the location of the alarm signal.
This list of mandated requirements for an electronic alarm device is quite demanding, but also very necessary in a complex medical setting. The search for the avoidance of “sonic ambiguity” in medical alarms led to the development of the IEC 60601-1-8 technical standard. This guideline helped to spell out the requirements for the design and test of alarms in medical electronic equipment and continues to evolve to meet the requirements of a rapidly expanding field.
As mentioned, CUI Devices’ offers a range of medical buzzers compliant with the alarm signal requirements of IEC 60601-1-8. This family of piezo audio indicators produces low, medium, and high priority tones for general medical use as well as tones for specific medical applications, including ventilator, oxygen, and cardiovascular equipment. CUI Devices’ line of medical speakers is also designed to meet IEC 60601-1-8 to help simplify medical design integration.
More Info
Designers of medical electronic devices or systems can purchase the IEC 60601-1-8 document on the webstore page of the IEC website at www.iec.ch. Additional information on the standard and designing alarms in medical electronic equipment can also be located through the Association for the Advancement of Medical Instrumentation (AAMI), the National Center for Biotechnology Information’s National Library of Medicine, or the Journal of the Acoustical Society of America (ASA). CUI Devices’ line of medical speakers is also designed to meet IEC 60601-1-8 to help simplify medical design integration.